eClinical Solutions Market Size To Reach $22.7 Billion By 2030
The global eClinical solutions market size is expected to reach USD 22.7 billion by 2030, growing at a CAGR of 14.1% from 2024 to 2030, according to a new report by Grand View Research, Inc. Increasing R&D activities by biopharma and pharma companies, application of software solutions in clinical trials, and expanding customer base are anticipated to fuel market growth. During COVID-19, clinical laboratories experienced high demand for COVID-19 tests. Clinical data management systems assisted these laboratories to seamlessly manage the huge influx of specimens daily. Technological advancements such as electronic data capture and Wi-Fi connectivity are projected to drive the market in the forthcoming years.
As the demand for tracking and analyzing clinical data increases, the need for effective clinical solutions rises. Unmet needs to manage efficient clinical development processes are poised to boost market growth over the forecast period. Moreover, digital transformation in the field of clinical trials and preference for data-centric approaches are providing a tremendous push to the market. Demand for integrated clinical IT solutions is increasing due to the massive volume of data generated during clinical development processes. eClinical solutions offer a single source of information that helps optimize the cost by eliminating redundant data entry and by reducing on-site verification and source data verification. Rising awareness regarding these advantages is projected to propel the market.
Increasing adoption of eClinical workflows in trials offers enormous potential in clinical development processes. These solutions can facilitate decision-making in each stage of development. It helps reduce cost and time between the development phase by utilizing seamless designs and identifying failing compounds. It offers rapid access to data and patient safety information, which helps make quick decisions. Market players engage in new product development and strategic alliances including partnership, agreement, promotional activities, etc. to keep market rivalry high. In August 2023, Sitero acquired Clarios eClinical technology suite to enhance clinical trial delivery. This acquisition demonstrates Sitero's dedication to remain at the forefront of innovation and offering its clients the best possible support and services.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/eclinical-solutions-market
eClinical Solutions Market Report Highlights
- Based on product, the CTMS segment led the market in 2023 and accounted for a revenue share of around 20.2% owing to benefits such as centralized end-to-end management of clinical trial activities, elimination of reliance on manual processes, real-time status tracking, and maintenance of multiple databases, which cumulatively improve the overall efficiency of clinical trials
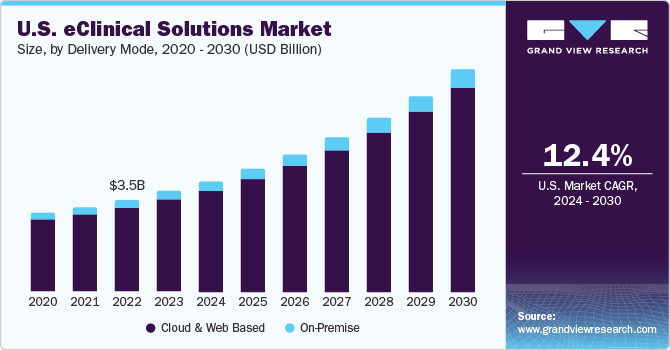
- Web and cloud-based systems are anticipated to exhibit an exponential CAGR during the forecast period owing to integrated features such as flexibility, high accessibility, negligible handling costs, and easy data backup. Real-time data is available through these systems, which enables users to take quick decisions and provide high-quality information for risk-based monitoring
- Based on development phase, the phase III segment held the largest market share of 53.3% in 2023. Phase I segment is expected to grow at the fastest CAGR over the forecast period
- Based on end-use, the CRO segment held the largest revenue share in 2023. The segment is projected to rise at a remarkable CAGR during the forecast period owing to the growing inclination of pharmaceutical companies to reduce overall expenditure.
eClinical Solutions Market Segmentation
Grand View Research has segmented the global eClinical solutions market based on product, delivery mode, development phase, end-use, and region:
eClinical Solutions Product Outlook (Revenue, USD Million, 2018 - 2030)
- Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Clinical Analytics Platforms
- Randomization and Trial Supply Management (RTSM)
- Clinical Data Integration Platforms
- Electronic Clinical Outcome Assessment (eCOA)
- Safety Solutions
- Electronic Trial Master File (eTMF)
- Electronic Consent (eConsent)
eClinical Solutions Delivery Mode Outlook (Revenue, USD Million, 2018 - 2030)
- Web and Cloud based
- On-premise
eClinical Solutions Development Phase Outlook (Revenue, USD Million, 2018 - 2030)
- Phase I
- Phase II
- Phase III
- Phase IV
eClinical Solutions End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Hospitals/Healthcare providers
- CROs
- Academic Institutes
- Pharma & Biotech Organizations
- Medical Device Manufacturers
eClinical Solutions Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- S.
- Canada
- Mexico
- Europe
- K.
- Germany
- France
- Italy
- Spain
- Netherlands
- Sweden
- Denmark
- Rest of Europe (EU) {RoE}
- Asia Pacific
- Japan
- China
- India
- South Korea
- Australia
- New Zealand
- Taiwan
- Hong Kong
- Singapore
- Thailand
- Vietnam
- Rest of Asia Pacific (RoAPAC)
- Central & South America
- Brazil
- Argentina
- Chile
- Rest of Latin America (RoLA)
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Egypt
- Qatar
- Rest of Middle East & Africa (RoMEA)
List of Key Players in the eClinical Solutions Market
- Fountayn, formerly known as Datatrak International, Inc.
- Oracle
- Calyx, formerly part of Parexel International Corporation
- Medidata (Dassault Systemes)
- CRF Health (Signant Health)
- Clario (ERT and Bioclinica)
- eClinicalWorks
- Merative (IBM Watson Health)
- Anju Software
- eClinical Solutions
- MaxisIT
- IQVIA
- Castor
- Veeva Systems
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/eclinical-solutions-market
Comments
Post a Comment