Pharmacovigilance Market Size, Share, Trends And Forecast 2030
The global pharmacovigilance market size is expected to reach USD 13.90 billion by 2030, according to a new report by Grand View Research, Inc. It is projected to expand at a CAGR of 9.3% from 2022 to 2030. The increasing incidence of Adverse Drug Reactions (ADR) is a key growth factor. ADR imposes a substantial burden on healthcare systems and is one of the prominent causes of morbidity in developed countries. According to the National Center for Biotechnology Information (NCBI), approximately 5% of total hospitalizations in Europe each year are due to ADR. Pharmacovigilance services play an integral role in this clinical trial phase by assisting manufacturers in identifying adverse effects associated with the drug.
COVID-19 has undoubtedly thrown up numerous challenges as well as opportunities for pharmacovigilance service providers. Various companies are developing innovative platforms to gain a competitive edge. For instance, in December 2020, ArisGlobal and EVERSANA declared a strategic partnership to digitally transform end-to-end pharmacovigilance services globally.
The community involved in PV procedure has been quick to respond to the pandemic. Some companies are using big data analytics against COVID-19. Thus, this refers to a depth analysis of data from multiple sources. In April 2020, Saama Technologies offered its Life Science Analytics Cloud technology platform to support the consortium creation. The purpose is to fetch data from both current and future studies to slash the time required to discover a treatment by as much as 50%. Life Science Analytics Cloud is an artificial intelligence-powered platform. This represents the scope for future developments in this market.
According to the World Health Organization's (WHO) report on pharmaceutical consumption, chronic disease medications accounted for a larger proportion of the total volume of drug consumption in non-hospital setups. Hence, there has been a significant rise in the number of medicines made available to healthcare consumers. The rising demand for drugs has significantly heightened the need for the development of novel therapeutics via extensive clinical trials, which is expected to serve this market with lucrative opportunities.
Moreover, leading pharma companies in developed countries are focusing on outsourcing PV services to reduce costs and minimize operational expenses. This is anticipated to provide an opportunity to contact research organizations in developing regions to gain more revenue share. Manufacturers are now focusing on remodeling their product development processes in an attempt to cater to patient needs across the globe. These factors are anticipated to fuel the demand for pharmacovigilance services during the forecast period.
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/pharmacovigilance-industry
The companies operating in the market are undertaking strategic initiatives, such as collaborations with PV service providers to gain access to medical information and manage PV workflows. For instance, In October 2021, The Whiteboard, an academy for training specialists in the clinical trials and drug development field, proclaimed a partnership with OviyaMedSafe, a worldwide drug safety service and pharmacovigilance consulting corporation.
Similarly, in September 2019, Accenture collaborated with Bayer to implement the company's INTIENT Clinical platform to simplify and speed up its drug development processes, thereby widening its business. The company collaborated with BioCelebrate in the past to develop a platform for aggregating and analyzing clinical information for improving the efficiency of drug development, thus enhancing its R&D capabilities. Such initiatives help companies maintain their position and thereby support the market growth.
Pharmacovigilance Market Report Highlights
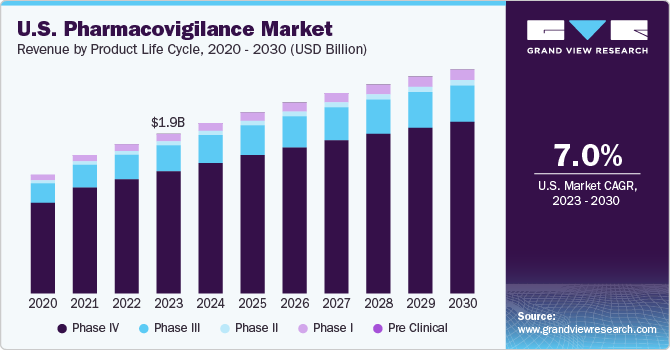
- By product life cycle, phase IV held a dominant share of over 75.0% in 2021 owing to the extensive post-marketing surveillance of pharmaceuticals and an increasing number of ADR incidences in the market
- By service provider, contract outsourcing held a significant share of over 55.0% in 2021 owing to the shift in the focus of pharmaceutical companies toward outsourcing services to reduce operational cost
- Based on type, spontaneous reporting held the largest revenue share in 2021 due to its wide application in pharmacovigilance and benefits such as easy simulation of data sets for better drug comparison
- The biotechnology companies end-use segment is anticipated to exhibit a lucrative CAGR over the forecast period owing to increasing R&D for the development of novel biologics
- Asia Pacific is anticipated to register a lucrative CAGR of 10.8% over the forecast period. This is attributed to the availability of low-cost labor and the rising number of outsourcing companies in this region
- Industry participants are focusing on increasing R&D activities to develop better pharmacovigilance services. Moreover, companies are adopting strategies including new product launches, collaborations, and mergers & acquisitions to gain a competitive advantage
Key Companies & Market Share Insights
The market is witnessing a significant boost owing to the patent expiration of branded drugs and the increasing number of new drug developments. This has attracted several local and international pharmacovigilance service providers. The presence of a competitive milieu has led to improved clinical data management and has streamlined the R&D process.
The market is competitive with key participants involved in continuous product development, collaborations, partnerships, and alliances to augment market penetration. For instance, in December 2019, Accenture and UCB announced the collaboration to accelerate data processing and help in improving patient safety, thereby widening their business in their respective markets. The rising number of Contract Research Organizations (CROs) and increased demand for outsourcing services are expected to further fuel the competition in near future. Some prominent players in the global pharmacovigilance market include: Accenture, Cognizant, Laboratory Corporation of America Holdings, IBM Corporation, ArisGlobal, ICON plc., Capgemini, ITClinical, FMD K&L, IQVIA, TAKE Solutions Ltd., PAREXEL International Corporation, BioClinica Inc., Wipro Ltd., United BioSource Corporation
Request Free Sample Report: https://www.grandviewresearch.com/industry-analysis/pharmacovigilance-industry
Comments
Post a Comment