Regulatory Affairs Outsourcing Market Worth $13.9 Billion By 2030 | Grand View Research, Inc.
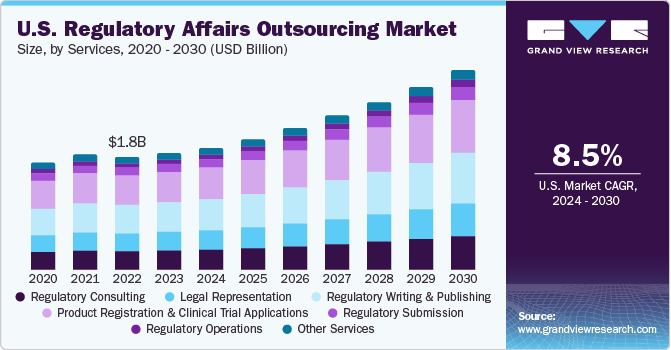
The global regulatory affairs outsourcing market size is expected to reach USD 13.9 billion by 2030, registering a CAGR of 8.9% over the forecast period, according to a new report by Grand View Research, Inc. Significant increase in the fixed costs of in-house resources for regulatory affairs & operation activities like training, technology, specialized knowledge, and facilities are driving outsourcing of regulatory affair functions. Addressing local regulatory challenges and constant changes in the regulations of the major markets, such as the U.S., Europe, and Asia, is creating demand for these services. Compliance with the current regulations has become an immense chore, leave alone trying to stay up to date with developments around the world. Amendments to current regulations are likely to simplify the regulatory pathway for the industry but in turn, complicate the operation for healthcare product manufacturing companies.
Thus, leading to the regulatory affairs
outsourcing to service providers. The market witnessed a significant decline in
2020 due to the outbreak of the COVID-19 pandemic, as there was a halt in
clinical trial activities including regulatory updates. Disruption in the
supply chain and closure of R&D sites resulted in a significant loss of
revenues in 2020 across the industry. However, due to the pandemic, various
healthcare companies are opting for more regulatory advice, as companies push
to speed up the product development & approvals in therapies, vaccines,
& devices for COVID-19. Owing to the urgent need for medical devices and
therapies for COVID-19, regulatory authorities are providing emergency use
authorization for the majority of products. Such actions have further promoted
the demand for these services post 2020 and the positive rebound of sales
across the industry can be witnessed from 2021.
On the basis of geographies, the market has
been further divided into North America, Europe, Asia Pacific, Latin America,
and Middle East & Africa. The Asia Pacific region accounted for the maximum
share of more than 38% of the global revenue in 2021. The region is also
projected to witness the fastest CAGR over the forecast period. This can be
attributed to the increasing number of clinical trials and the rising number of
companies trying to enter markets in developing countries, such as India and China.
Furthermore, the availability of a skilled workforce in the region at lower
costs compared to the U.S. is another factor expected to propel the regional
market growth.
The North America regional market also
reported a significant share in the global industry. The presence of key
pharmaceutical and medical devices companies and the rise in the R&D
spending in the region and some of the key factors driving the market in North
America. North America and Europe are expected to be the key markets for regulatory
affairs outsourcing owing to the presence of two major international regulatory
agencies, the European Medicines Agency (EMA) and U.S. FDA, which regulate more
than half of medical devices worldwide.
Comments
Post a Comment